The first step we have to follow is to convert the units of each of the measurements (mL to L, mmHg to atm and °C to K):
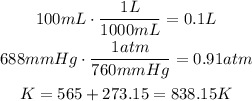
Now, use the ideal gases law to find the number of moles of carbon dioxide at these conditions:
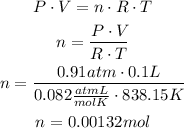
Now, use the chemical equation to find how many moles of acetic acid are needed to produce that amount of carbon dioxide:
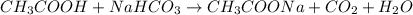
Use the stoichiometric ratio of moles of acetic acid used to moles of carbon dioxide produced:
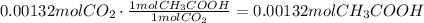
Use the molar mass of acetic acid to convert this amount of moles to mass:
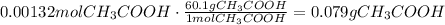
Finally use the density of acetic acid to find volume of 0.079g of this compound:
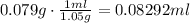
It means that 0.08292 would be needed to obtain the sample of carbon dioxide.