To find the answer to this question, the first step is to find the limiting reactant. To do it, we have to convert the given masses to moles using the molecular mass of each reactant.
The molecular mass of the FeO is 71.84g/mol and the one of the Al is 26.98g/mol.
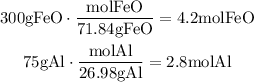
Now, using the mole ratio of the reactants we can determine the limiting reactant:
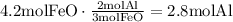
According to this, none of them is the limiting reactant since the amount of FeO available is the required for the amount of Al available. Use the mole ratio of FeO to Fe to find how many moles of Fe will be produced:
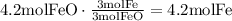
Use the molecular mass of Fe to find the mass of Fe produced in grams:
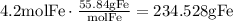
The answer is 234.528grams of Fe.