The first step is to convert the kPa to atm, using a conversion factor:
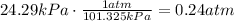
Also convert the ml to L, using a conversion factor:
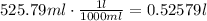
Use the ideal gas law to find the temperature of the gas:
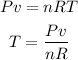
Where T is the temperature, P is the pressure, v is the volume, n is the number of moles and R is the ideal gas constant (0.082atm*L/mol*K):
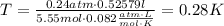
The answer is 0.28K.