Answer:
37,555.584 Joules
Explanations
The formula for calculating the amount of energy needed is expressed as:
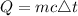
where:
• m is the ,mass, of water = 136grams
,
• c is the ,specific heat, capacity of water = 4.184 J/g°C
,
• △t is the change in ,temperature, = 78 - 12 = 66°C
Substitute
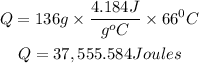
Hence the amount of energy that is needed is 37,555.584 Joules