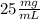A 2.5% solution means that we have:
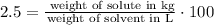
This basically means that:
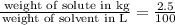
But we are being asked about mg/mL so we need to trnasform kg and L into mg and mL. We know that:
![\begin{gathered} 1L=1000mL \\ 1\operatorname{kg}=1000g=1000000mg \end{gathered}]()
If we use this in the former equation we have:
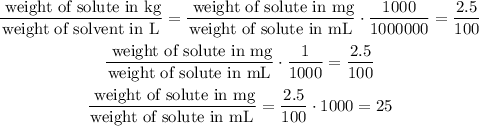
Which basically means that this solution has 25mg of solute per each mL of solvent so the answer is: