Since we already have the number of moles of He, we can discover how many atoms of He there are by using the relationship between moles and number of atoms, i.e.:
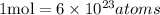
Since, in this case, the number of moles of He is 0.0025 moles, we can set the following proportion to discover the number of He atoms:
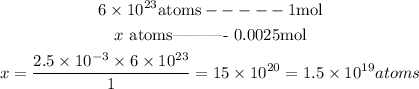
So, the number of He atoms in 0.0025 moles of He is 1.5x10^(-19) atoms.
Important remember: in this exercise, we did not have to use the volume because we already had the equivalent in moles. If we had only the volume, then we could use the molar volume at STP, 22.4 L/mol, to calculate the amount of moles.