The formula for the pH is the following:
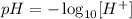
Where the concentration is in mol L⁻¹.
So, if we want the formula to calculate the concentration given the pH, we have to invert it:
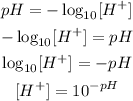
Now, let's answer the items:
(a) It turns red for pH 4.4 or lower. So:
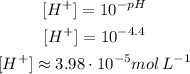
The methyl red turn red for a concentration of H⁺ of approximately 3.98 10⁻⁵mol L⁻¹ or higher.
(b) It turns yellow for pH 6.2 or higher. so:
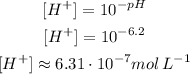
The methyl red turn yellow for a concentration of H⁺ of approximately 6.31 10⁻⁷mol L⁻¹ or lower.
(c) In a concentration of H⁺ of 3.2 10⁻⁴mol L⁻¹, we have:
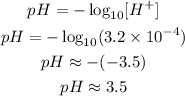
Which is lower then 4.4, so it will turn red.