Answer : Option A , 33.7 moles
Solution:
We are given :
• Mass of Ca(OH)2 = 2.5 kg = 2.5kg *1000g/kg = ,2500 grams,.
Step 1:
Calculate Molecular Mass of Ca(OH)2 = 74.09268 mol/g, calculated as follows :
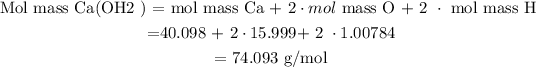
Recall that Molecular mass of a substance is obtained from the periodic table .
Step 2 : Use the formula : moles = mass /molecular mass , to calculate number of moles of Ca(OH)2:
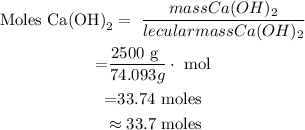
This means that there is 33.7 moles in 2.5kg of Ca(OH)2