The Ideal Gas Law formula is:
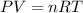
In the formula:
P is the pressure of the gas.
V is the volume of the gas.
n is the number of moles.
R is the gas constant.
T is the temperature of the gas.
So, to analyze how temperature and volume change as the other is increased or decreased we uses the Charles's Law. The Charles's Law is one of the experimental gas laws that helped to create the Ideal Gas Law, and the one that relates volume and temperature.
According to the Charles's Law, when we have a certain amount of gas at constant pressure, as the temperature increases, the volume of the gas increases and as the temperature decreases, the volume of the gas decreases.
This happens because the variables, volume and temperature, are directly proportional in the formula of the Ideal Gas Law.