Answer
The concentration of strontium hydroxide = 0.11 M
Step-by-step explanation
Given:
Volume of strontium hydroxide, Vb = 45 mL
Volume of chloric acid, Va = 32 mL
Concentration of chloric acid, Ca = 0.30 M
What to find:
The concentration of strontium hydroxide, Cb.
Step-by-step solution:
Step 1: Write a balanced equation for the neutralization reaction.
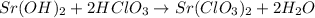
Mole ratio from the equation is 1:2, that is nb = 1 and na = 2
Step 2: Determine the concentration of strontium hydroxide, Cb.
Using the formula below;
CaVa/na =CbVb/nb
So Cb = (Ca x Va x nb)/(Vb x na)
Plugging the values of the given parameters into the equation, we have
Cb = (0.30M x 32mL x 1)/(45mL x 2)
Cb = 9.6 M/90 m
Cb = 0.11 M
The concentration of strontium hydroxide = 0.11 M