Answer:
Percentage of zinc = 50.81%
Percentage of Phosphorous = 16.05%
Percentage of oxygen = 33.14%
Explanations:
The molecular formula of zinc phosphate is given as:
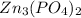
To get the percentage of each element in the compound, we will apply the formula:
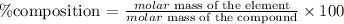
Get the molar mass of the compound.
Given the molar masses of the elements
Zinc = 65.38g/mol
Phosphorous = 30.97g/mol
Oxygen = 15.99g/mol
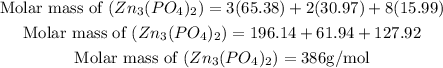
Get the percentage composition by mass of each element:
For Zinc element;
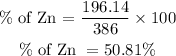
For Phosphorous element
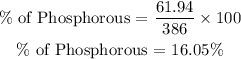
For the oxygen element;
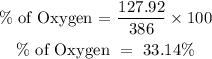
Therefore the percentage composition of Zinc, Phosphorous, and Oxygen in zinc phosphate is 50.81%, 16.05%, and 33.14% respectively