Answer
20.8 moles of H₂O
Step-by-step explanation
Given:
The moles of iron(III) oxide produced = 5.2 mol
Equation: 3Fe(s) + 4H2O(l) ⇨ Fe3O4(s) + 4H2(g)
What to find:
The moles of water required to produce 5.2 moles of iron(III) oxide.
Solution:
Using the mole ratio of water to iron (iii) oxide in the given equation; which is
4 moles of H₂O produced 1 mole of Fe₃O4
So, x moles of H₂O will produce 5.2 moles of Fe₃O4
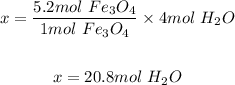
Hence, the moles of water required to produce 5.2 moles of iron(III) oxide is 20.8 moles