The first step to solve this problem is using the molecular mass of ethyleneglycol to find the mass of 0.350moles of this compound.
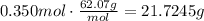
Use the percent by mass to find the mass of the solution, remember that there are 56g of ethyleneglycol for every 100g of solution:
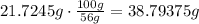
Use the density of the solution to find how many mililiters contain the estimated mass of solution :
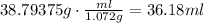
The volume of solution that contains 0.350 moles of ethylene glycol is 36.18ml.