Answer:
1.0535 * 10²⁴ molecules
Explanations:
Given the following parameters
Moles of water = 1.75 moles
According to Avogadro's constant:
1 mole = 6.02 * 10²³molecules
The number of molecules of water in 1.75 mol of water is calculated as:
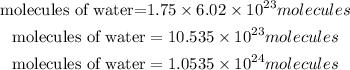
Hence the number of water molecules in 1.75 moles of water is 1.0535 * 10²⁴ molecules