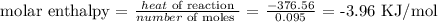Answer:
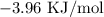
Step-by-step explanation:
Here, we want to get the molar enthalpy of the solution
We start by getting the change in enthalpy
Mathematically, we have that as follows:
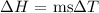
where:
m is the mass of water (we can get this by converting the volume given to liters and multiplying by 1000 g. This is same as 75 g)
Mathematically, this can be done as follows:
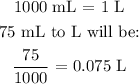
Now, to convert this volume to mass, we multiply the volume above by 1000 g/L.
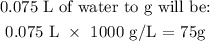
s is the specific heat capacity of water which is 4.184 J/g °C
Delta T is the change in temperature which is (23.7 - 24.9) = -1.2°C
Substituting the values, we have it that:
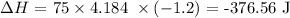
To get the molar enthalpy of the solution, we have to divide the heat of reaction calculated above by the number of moles
The number of moles can be calculated by dividing the mass by the molar mass of sodium chloride
The molar mass of sodium chloride is 58.5 g/mol
The number of moles will be:
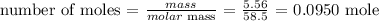
We now proceed to divide the amount of heat by this number of moles as follows: