Given,
The distance between the charges is r=5×10⁻² m
From Coulomb's law, the force between two charges is given by,
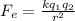
Where k is the coulomb's constant, q₁ and q₂ are the charges.
The magnitude of the charge of proton and electron is q=1.6×10⁻¹⁹ C
Thus the force between the two electrons and two protons is,
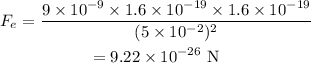
Thus the force between two electrons and two protons respectively is 9.22×10⁻²⁶ N