Answer:
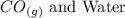
Step-by-step explanation:
Here, we want to get the products of the incomplete combustion of methane
For the incomplete combustion of methane, we have methane being burned in a limited supply of oxygen
Due to the insufficient supply of oxygen, the oxide of carbon that would be formed is Carbon (ii) oxide, alongside water
If oxygen was sufficient, it means that combustion will be complete and the oxide of carbon formed alongside water will be Carbon (ii) oxide