Answer:
There are 0.0753 moles of KBr.
Step-by-step explanation:
The molarity of the solution tells us that there are 1.50 moles of KBr in 1000mL of solution.
But we only have 50.2mL of solution, so with a mathematical rule of three we can calculate the amount of moles in 50.2mL:
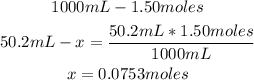
So, there are 0.0753 moles of KBr.