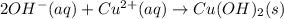Answer
The general equation for the reaction is:
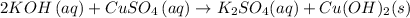
The ionic equation for the reaction is:
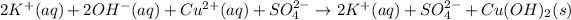
Step-by-step explanation
Given unbalanced equation: KOH(aq) + CuSO4(aq)
The general equation for the reaction is:
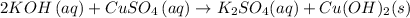
The reaction type is a double displacement reaction.
The ionic equation for the reaction is:
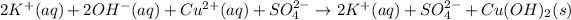
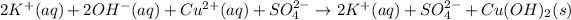
Cross out the spectators' ions on both sides of the complete ionic equation to get the net ionic equation as shown below: