Answer:
0.608 moles are in 13.8L.
Step-by-step explanation:
At STP conditions, the molar volume of a an ideal gas it is always 22.7L.
So, with a mathematical rule of three we can calculate the moles of neon contained in 13.8L:
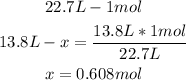
Finally, 0.608 moles are in 13.8L.