1. The first thing we do is chech if the reaction is balanced:
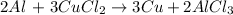
As we can see the equation is balanced because there are on both sides:
2 atoms of Al
3 atoms of Cu
6 atoms of Cl
2. Now we calculate the moles of each reactant that we have:
For this we need to know the molar mass of each reactant. We calculate it using the periodic table.
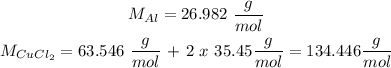
So we calculate:
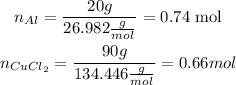
3. Now we calculate the limiting reactant:
If we consider that all the aluminum reacts, for 0.74 moles we would need:
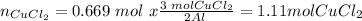
So we see that we need 1.11 moles of CuCl2 in order for the aluminum to react completely. So the limiting reactant is CuCl2.
Now we calculate the amount of copper produced:
So the amount of copper produced is 0.669 moles.