The reaction is:
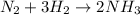
So for every molecule of nitrogen we need 3 molecules of hydrogen.
Now, we have 4 molecules of nitrgen, so if the total amount of nitrogen react we would need 4x3=12 molecules of hydrogen.
We know that there are only 9 molecules of hydrogen present, so it is not possible that all the nitrogen present react. Therefore the limiting reactant is hydrogen.
To answer how many molecules of ammonia (NH3) are produced we need to calculate the amount formed if all the hydrogen present reacts:
For every 3 molecules of hydrogen 2 molecules of ammonia are formed, so for 9 molecules of hydrogen the molecules of ammonia formed are: 2x3=6.
To form 6 molecules of ammonia there are needed 6/2=3 molecules of nitrogen, so only one remains after the reaction.
To summerize:
• The number of molecules of ammonia formed are 6
,
• The limiting reactant is hydrogen
,
• The number of molecules remaining after the reaction are:
molecules of hydrogen: 0
molecules of nytrogen: 1