Answer
We have the following chemical reaction:
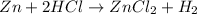
We can see that the equation is balanced because we have the same number of each atom in both sides of the equation.
Now, as we can see in the equation, for every mol of Zn that reacts it is needed 2 moles of HCl. Therefore we can calculate the moles of HCl needed in total as follows:
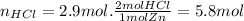
So the answer is: there are needed 5.8 mol.