ANSWER
Weak acid
Step-by-step explanation
Firstly, we need to differentiate between weak acid and strong acid
Strong acid is a type of acid that ionize completely in an aqueous solution
Weak acid is a type of acid that ionize partially in an aqueous solution.
CH3COOH which is also known as acetic acid dissociate partially in an aqueous solution
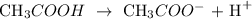
Therefore, CH3COOH is a weak acid