Step 1 - Briefly remembering how to balance an equation
To balance a chemical equation, we must guarantee that the number of atoms of the same element is the same in both sides of the equation.
In order to do so, we need to adjust the coefficients, always remembering that the coefficients multiply the smaller numbers in the formula of each substance.
Step 2 - Balancing the given equation
The given equation is:
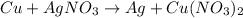
Note that there are two NO3 in the right hand side. So we need to multiply AgNO3 by 2 as well:
![Cu+2AgNO_3\operatorname{\rightarrow}Ag+Cu(NO_3)_2]()
Now we also need to adjust Ag, by multiplying it by two, in order to have 2Ag in each side:
![Cu+2AgNO_3\operatorname{\rightarrow}2Ag+Cu(NO_3)_2]()
The equation is now properly balanced.
The coefficients are thus: 1,2,2,1