Answer:
Step-by-step explanation:
The energy of a quantum of light with frequency v is given by
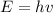
where h = planck's constant = 6.63 *10^-34 and v = frequency of light.
Now in our case, we are told that
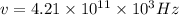
The extra 10^3 comes from the fact that we were given kilohertz (and not simply hertz).
Substituting the value of the given frequency and the Planck's constant into the above equation gives
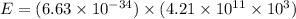
which upon evaluating gives
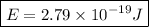
Hence, the energy of our quantum of light is 2.79*10^-19 Joules!