1) Chemical equation
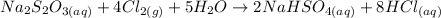
2) Moles of Na2S2O3 needed to react with Cl2 (a).
The molar ratio
1 mol Na2S2O3: 4 mol Cl2
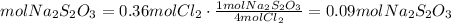
(a) We need 0.09 mol Na2S2O3 to react with 0.36 mol Cl2.
3) Moles of HCl formed from Cl2 (b).
The molar ratio
8 mol HCl: 4 mol Cl2
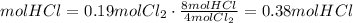
(b) 0.19 mol Cl2 can form 0.38 mol HCl
4) Moles of H2O required for the reaction of Cl2 (c).
The molar ratio
5 mol H2O: 4 mol Cl2
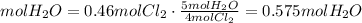
(c) 0.575 mol H2O are required for the reaction of 0.46 mol Cl2.
5) Moles of H2O to form HCl
The molar ratio
5 mol H2O: 8 mol HCl
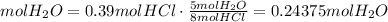
(d) 0.24375 mol H2O is needed to form 0.39 mol HCl.