Answer;
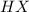
Step-by-step explanation:
Here, we want to get the stronger acid here
The reaction favors the product as given in the question.
The equilibrium will always favor the side where we have the weaker acid
For the product to be favored, it simply means that HY is the weaker acid
Thus, in that case, HX will be the stronger of the two acids