Answer:
15.9 g CO2.
Step-by-step explanation:
What is given?
Mass of liquid hexane ( CH3(CH2)4CH3 ) = 5.17 g.
Mass of oxygen gas (O2) = 34.1 g.
Molar mass of hexane = 86 g/mol.
Molar mass of O2 = 32 g/mol.
Molar mass of CO2 = 44 g/mol.
Step-by-step solution:
First, let's state the balanced chemical equation:
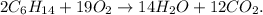
Now, let's calculate the number of moles of each reactant using their respective molar masses.
For hexane (C6H14):
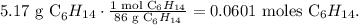
And for O2:
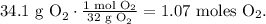
Let's see how many moles of carbon dioxide are being produced by each reactant.
In the chemical equation you can see that 2 moles of C6H14 reacted produces 12 moles of CO2:
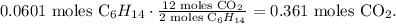
And in the chemical equation you can see that 19 moles of O2 reacted produces 12 moles of CO2:
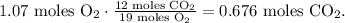
You can realize that the limiting reactant is hexane (C6H14) because this reactant is being consumed first and we can produce only 0.361 moles of CO2. There is an excess of O2.
The final step is to calculate the mass of CO2 produced using its molar mass, like this:
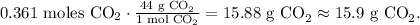
The answer is that the theoretical yield of carbon dioxide is 15.9 g CO2.