The formula to find the area of a circle is:
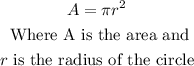
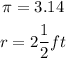
As the radius of the circle is a mixed fraction then we are going to convert it to an improper fraction and thus be able to work more easily with this number:
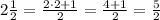
Now, you have:
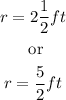
And then:
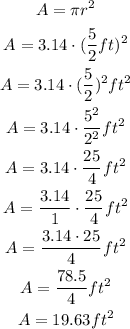
Therefore, the area of this circle will be 19.63 square feet.