Answer:
Temperature increases. Option A is correct.
Explanations:
According to charles law, volume of a given mass of gas is directly proportional to absolute temperaturee provided that the pressure is constant.
Mathematically, we can have:
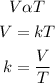
According to the equation, we can see that as the volume of the substances decreases/increases, the temeperature also decreases/increases.
From the explanations above, we can conclude that as the volume of a system increases, the temperature also increases.