Given:
The amount at 120 secs and 200 secs is respectively,
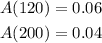
To find: The average rate of the reaction from 120 to 200 seconds.
Step-by-step explanation:
Using the average rate of change formula,
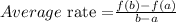
Here, a = 120 and b = 200.
Substituting the above values we get,
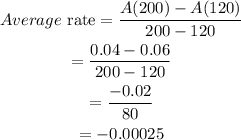
Final answer:
The average rate of the reaction is -0.00025mol/L/sec.