Because we are looking for the temperature change caused by a volume change at constant pressure, this is for Charles’s law. Taking V1 and T1 as the initial values, we can use the following equation:
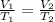
We are given:
T1 = 300 K
V1 = 3 L
V2 = 10 L
(3L/300K) = (10L/T2)
T2 = 10L/(3L/300K)
T2 = 1000 K)