Answer:
• Atom B
,
• Atom R
,
• Atom A
,
• Atom P
Step-by-step explanation:
To calculate the overall charge of each atom, it is necessary to do a subtraction between the number of protons and the number of electrons:
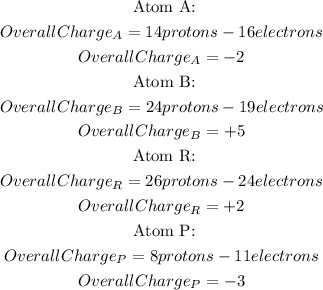
So, the order of the atoms from most positive to least positive is:
• Atom B
,
• Atom R
,
• Atom A
,
• Atom P