Answer:
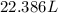
Explanations:
In order to get the required volume of the gas, we will use the ideal gas equation expressed as:
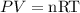
P is the pressure of the gas (in atm)
V is the volume of the gas (in L)
n is the number of moles
R is the Gas constant
T is the temperature of the gas
Given the following parameters:
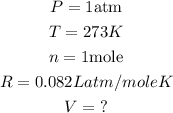
Substitute the given parameters into the formula to get the volume:

Hence the volume of a gas at 273K be if I have 1 mole of the gas at 1 atm is 22.386L