We know that the atomic mass of Cl-35 is 34.969 amu and its abundance is 75.5%. The atomic mass of Cl-37 is 36.966 amu and its abundance is 24.5%.
To find the average atomic mass, first, we multiply each percentage by the atomic mass of each isotope.
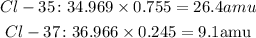
Then, add them.
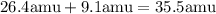
Observe that the atomic mass we found is closer to the isotope Cl-35, this is because this isotope has a greater percentage of occurrence in nature.
Therefore, its atomic mass is 35.5 amu.