To convert mass (g) and number of moles, we can always use the molar mass of the substance, which can be found in the periodic table. For Zinc (Zn), the molar mass is 65 g/mol, which means that each 65 g of Zn corresponds to 1 mol.
So, if we want to discover how many moles of Zinc there are in 32g of Zn, we can set the following proportion:
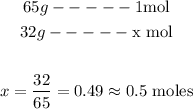
So, in 32g of Zinc (Zn), we have 0.5 moles.