Answer
P1 = 0.466 atm
Step-by-step explanation
Given:
Initial volume (V1) = 154.3 L
Final volume (V2) = 50.0 L
Initial Temperature (T1) = 27 °C = 300 K
Final Temperature (T2) = 40.0 °C = 313 K
Initial pressure (P1) = ?
Final pressure (P2) = 1.5 atm
Required: Initial pressure (P1)
Solution
To solve this problem, we will use combined gas law:
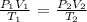
Re-arrange:
P1 = (P2V2T1)/T2V1
P1 = (1.5 x 50.0 x 300)/(313 x 154.3)
P1 = 0.466 atm