Answer:
-6.15
Explanations
The formula for calculating the pH of a buffer is expressed as
![pH=pka+log([salt])/([acid])^](https://img.qammunity.org/2023/formulas/chemistry/college/zknj49srzfwgridi6uaemfed00fakz9cbp.png)
pka of the hydrochloric acid = -6.3
Calclate the moles of the HCl and sodium acetate
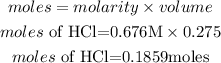
Calculate the moles of sodium acetate (salt)
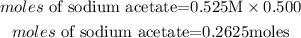
Substitute the given parameters into the formula
![\begin{gathered} pH=pka+log\frac{[0.2625\text{/v}]}{[0.1859\text{/v}]} \\ pH=-6.3+log1.4121 \\ pH=-6.3+0.1498 \\ pH=-6.1502 \end{gathered}](https://img.qammunity.org/2023/formulas/chemistry/college/onvg9veewer24beka9rradlxswy24lqb2b.png)
Hence the pH of this buffer is approximately -6.15