Charle's Law states that the volume varies directly with it's temperature and can be expressed as :
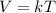
Where V is the volume, T is the temperature and k is some constant
From the given problem, we have :
V1 = 0.8 liters
T1 = 400 degrees
V2 = 0.3 liters
Since k is a constant,


We can express both equation as k = V/T
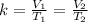
Substitute the given values to the formula :
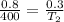
Then solve for the value of T2 :
Simplify the equation by multiplying 400T2 to both sides of the equation :
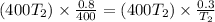
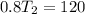
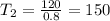
Therefore the answer is 150 degrees