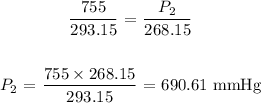Answer:
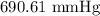
Step-by-step explanation:
Here, we want to get the resulting pressure
According to the pressure law, temperature and pressure are directly proportional
Mathematically:
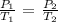
where:
P1 is the initial pressure which is 755 mmHg
P2 is the final pressure which is not given
T1 is the initial temperature which we will convert to Kelvin by adding 273.15 K : 20 + 273.15 = 293.15 K
T2 is the final temperature which is -5 + 273.15 = 268.15 K
Substituting the values, we have it that: