Answer:
148.44psi
Explanations:
To solve this question, we will use Boyle's law that states that "the pressure of a given mass of gas is inversely proportional to the volume provided that the temperature is constant". Mathematically;
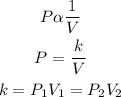
• P₁ and P₂ are the, initial ,and, final pressure, of the gas
,
• V₁ and V₂ are the ,initial, and, final volume ,of the gas
Given the following parameters:
P₁ = 64psi
V₁ = 6.958L
V₂ = 3L
Required
New pressure of the gas P₂
Substitute the given parameters into the formula;
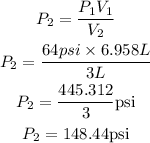
Therefore the new pressure of the gas in psi is 148.44psi