Answer: 7.02 x 10^24 molecules of methane correspond to 11.7 moles of this compound.
Step-by-step explanation:
The question requires us to calculate the number of moles of methane (CH4) that correspond to 7.02 x 10^24 molecules of this compound.
We can use the Avogadro's number to solve this problem: this proportionality constant tells us that there are 6.022 x 10^23 particles (ions, atoms, molecules etc.) in 1 mol of any compound.
Thus, we can write:
6.02 x 10^23 molecules CH4 ---------------- 1 mol CH4
7.02 x 10^24 molecules CH4 ----------------- x
Solving for x, we'll have:
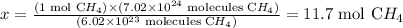
Therefore, 7.02 x 10^24 molecules of methane correspond to 11.7 moles of this compound.