Answer
The percent by mass of hydrogen in aspirin, C9H8O4 = 4.44%
Step-by-step explanation
What to find:
The percent by mass of hydrogen in aspirin, C9H8O4
Step-step-solution:
The first step is to determine the mass of hydrogen in aspirin.
From the periodic table, the atomic mass of H = 1.0 g
Since there are 8 atoms of H in aspirin, the mass of H in C9H8O4 will be
8 x 1.0 g = 8.0 grams
The next step is to determine the total mass of aspirin.
From the periodic table, the atomic masses: (H = 1.0, C = 12.0, O = 16.0)
Total mass of C9H8O4 = (9 x 12.0 g) + (8 x 1.0 g) + (4 x 16.0 g)
Total mass of C9H8O4 = 108.0 g + 8.0 g + 64.0 g
Total mass of C9H8O4 = 180.0 grams
The final step is to determine the percent 6by mass o hydrogen in aspirin, C9H8O using the formula below:4
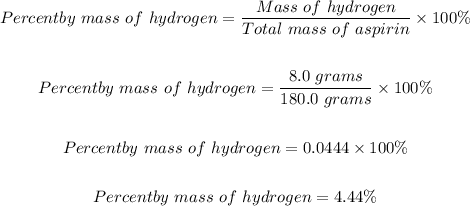
The percent by mass of hydrogen in aspirin, C9H8O4 = 4.44%