Similar shapes
Initial explanation
We know that the division of the corresponding sides of two similar shapes has always the same result.
In this case the corresponding sides are the same colored sides:
Then, we have that:
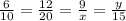
Finding x
In order to find x we are going to choose two fractions of divided corresponding sides:
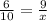
Now, we solve the equation:
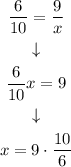
And we can obtain x value:
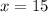
Finding y
In a similar way as before, we choose two of the divisions of corresponding sides:
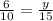
And we solve the equation:
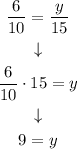
Answers:
x = 15 and y = 9