Step 1 - Understanding what happens to the concentration of the solute when it is diluted
The dilution process consists of adding more solvent to a previous solution. Let's say we have a solution containing salt (the solute) and water (the solvent).
When we add more water, the same amount of salt will be distributed over a large solvent volume.
Remember that concentration can be expressed as:
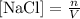
In this formula, n indicates the number of moles, while V indicates the volume of the solution. When the volume increases (because we add more water, for example) the concentration decreases.
Step 2 - Understanding pH
pH is a scale to measure acidity. It measures acidity by measuring the concentration of a specific cation, H+.
It may sound strange, but the pH has an inverse relation with the concentration of H+: the greater the concentration of H+, the lower the pH. This is a consequence of the definition of pH:
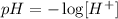
Step 3 - Putting it all together to solve the problem
We already know the soda has a pH of 4, which means it has a certain concentration of H+. Note that the soda is diluted in a 1:10 dilution. If the volume is increased tenfold, the concentration will be divided by 10.
Therefore, after the dilution, the new solution would be 10 times less concentrated in H+ than the original soda. As a consequence, the pH would go up.
The only possible solution thus is item d, the pH will go up as the H+ decreases due to solution.