Answer:
38.9mL are needed.
Step-by-step explanation:
1st) It is necessary to calculate the number of moles of HCl that will be contained the 161.1mL of the 3.03M solution:
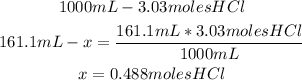
Now we know that the new solution will have 0.488 moles of HCl.
2nd) With the number of moles and the molarity of the original solution, we can calculate the volume of the 12.56M HCl needed:
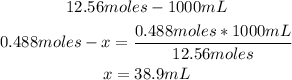
So, 38.9mL are needed.