Given data:
mass of sodium (Na) = 10.0 g
Chemistry -> The Mole -> Conversions Between Moles and Mass
Sodium (Na) is an element with an atomic number of 11 and an atomic mass of 23.0g. Sodium metal is an atom of sodium in its neutral state.
To convert 10.0 g of Sodium to moles, we must use its Molar Mass, which is the mass of the atom in one mole.
In one mole of Sodium, there are 23.0 g, since that is its atomic mass. Now we can do the conversion with the following relation:
23.0 g ------- 1 mole
10.0 g ------- x mole
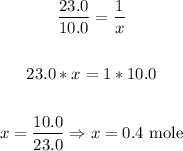
There are 0.4 moles of sodium metal in 10.0 grams.