1) Convert moles of Al to moles of S (a).
The ratio of Al to S in the compound is 2 Al: 3 S.
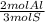
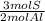
2) Convert moles of Al2(SO4)3 to S (b).
The ratio of S to the Al2(SO4)3 is 3 S: 1 Al2(SO4)3.
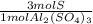
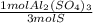
3) Moles of Al in the sample if the sample contains 1.97 mol S (c).
Convert moles of S to moles of Al.
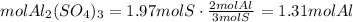
There is 1.31 mol Al in the sample.
4) Moles of S in 1.71 mol Al2(SO4)3
Convert moles of Al2(SO4)3 to moles of S
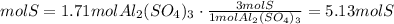
There is 5.13 mol S in the sample