We must balance the reaction equation before calculating moles. The C and hydrogen atoms are balanced since we have the same amount on each side of the reaction.
The oxygen is not balanced. We have 14 oxygens on the product side, so we have to put the coefficient 7 in front of the O2 molecule to get the same amount. So, the balanced equation of the reaction is:
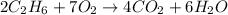
By stoichiometry, we see that the ratio CO2 to O2 is 4/7. So, we have to use this ratio to find the moles of CO2 that can be produced from 29 moles of O2. So, we have:
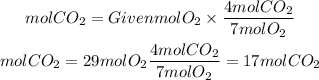
If you have 29 moles of O2 you can produce 17 mol of CO2.
Answer: 17 mol of CO2